
Late Breaking News
UPDATE FROM ABEONA
September 17, 2019
Abeona Therapeutics announce an open-label clinical trial assessing the safety of one-time gene therapy ABO-102 for patients with middle and advanced phases of MPS IIIA.
The official ClinicalTrials.gov listing for study ABT-003 in Patients With Middle and Advanced Phases of MPS IIIA Disease has been posted: https://bit.ly/2kkp9tA. The corporate website also has patient information about the study: https://bit.ly/2kIp9Ul.
July 9, 2019
Clinical Trials Accepting Eligible Children with Sanfilippo Syndrome type A and type B
Abeona Therapeutics Inc. is developing one-time gene therapies for Sanfilippo syndrome types A and B, also known as MPS IIIA and MPS IIIB. The Company is enrolling eligible children into two studies of these therapies, respectively – The Transpher A Study (for MPS IIIA) and The Transpher B Study (for MPS IIIB).
The best way to determine if your child may be eligible for one of these studies is to take a 6-question survey at AbeonaTrials.com. If the survey indicates your child may be eligible to enroll, you have the option of having the information sent to the nearest study site for evaluation by a study investigator – a doctor overseeing the clinical trial.
Importantly, only they can decide if your child may qualify for additional screening for the study and taking the survey does not mean a child is eligible.
If you agree to share the survey results, the study site will then contact you for more information. Survey results can also be downloaded to share with your child’s physician. Abeona does not have access to any patient information that is shared in this process.
If your child is enrolled in the study, reasonable costs of travel and meals needed by you and your child will be reimbursed, and there is no cost to you or your family to participate in the study.
If you think your child may be eligible or if you have questions about the study, email patients@abeonatherapeutics.com. You can also consult this FAQ.
Information for Your Child’s Doctor
Your child’s doctor can find more information about the studies at Clinicaltrials.gov using identifiers NCT02716246 and NCT03315182. Each study is evaluating the safety and efficacy of a single-dose gene therapy that delivers a functional copy of the defective SGSH or NAGLU gene. The therapies, ABO-102 and ABO-101, are administered through a single intravenous infusion of an AAV9-based vector for correction of the underlying enzymatic defect in each type of the disorder. Comprehensive information about each trial, including the full enrollment criteria, can be found at Clinicaltrials.gov.
Facebook Ad Campaign and Survey Aims to Help Enroll More Patients in Clinical Trials
Abeona recently launched a concerted effort to enroll more children into ongoing studies of its one-time gene therapies for MPSIIIA and MPSIIIB. The centerpiece is a Facebook advertising campaign and eligibility survey. The latter is intended to help caregivers determine if their child may be able to participate in one of the studies that are enrolling patients 6 months – 2 years of age. Children over 2 must have a development quotient of 60 or above to qualify. Full enrollment criteria is at Clinicaltrials.gov: MPSIIIA, MPSIIIB.
If the 6-question survey indicates a child may be eligible to enroll, the caregiver has the option of having the information sent to the nearest study site for evaluation by the investigator. The study site then engages the family for more information. Survey results can also be downloaded to share with a physician. Abeona does not have access to any patient information that is shared in this process.
Secondly, to bring more awareness to the studies and make them easier to decipher, Abeona has named them The Transpher A Study and The Transpher B Study. These names acknowledge the gene transfer method used by each therapy and the type of Sanfilippo syndrome they aim to treat.
More information about the therapies can be found on the company’s website or by contacting patients@abeonatherapeutics.com.
Abeona Therapeutics continues to show progress and bring hope to families. Its work is continually updated with full press releases here.
Abeona Therapeutics Reports Top-Line Data from Phase 1/2 Gene Therapy Trial in MPS IIIA
Abeona Therapeutics Reports Top-Line Data from Phase 1/2 Gene Therapy Trial in MPS IIIA
• ABO-102 results presented at WORLDSymposium for Lysosomal Diseases show significant time- and dose-dependent reduction of underlying disease pathology, including decreased CSF and urine GAGs (HS fragments) and diminished liver volumes
• Evidence of cognitive benefit at six months post treatment in Cohort 2 and at one year in Cohort 1
• Company receives FDA allowance to lower enrollment age to six months
• Company to host investor conference call Friday, February 9th at 9:00 am ET; dial in 877-407-9210 (toll free domestic) or 201-689-8049 (international)
NEW YORK and CLEVELAND, Feb. 08, 2018 (GLOBE NEWSWIRE) — Abeona Therapeutics Inc. (NASDAQ:ABEO), a leading clinical-stage biopharmaceutical company focused on developing novel cell and gene therapies for life-threatening rare genetic diseases, today announced updated clinical data from the ongoing Phase 1/2 trial for ABO-102 (AAV-SGSH), the company’s investigational gene therapy for the treatment of Sanfilippo syndrome Type A (MPS IIIA), a rare autosomal-recessive lysosomal storage disease. The results demonstrate robust and durable clinical effects achieved throughout various timepoints post-administration. To date, 10 patients have been dosed with a single intravenous injection of ABO-102. Results were reported today during the WORLDSymposium for Lysosomal Diseases being held this week in San Diego, CA.
“MPS IIIA is a profound and deadly lysosomal storage disease with no approved treatments available. The encouraging clinical data reported today provide strong additional support for a whole-body treatment approach involving intravenous delivery of an AAV to drive expression of the SGSH enzyme in all organs of the body, with an emphasis on expression in the central nervous system,” stated Kevin M. Flanigan, M.D., principal investigator of the trial, director of the Center for Gene Therapy at Nationwide Children’s Hospital and professor of pediatrics and neurology at The Ohio State University College of Medicine. “We are especially pleased to see sustained decreases in CSF heparan sulfate in all subjects post-injection, along with positive signals of neurocognitive activity in Cohorts 1 and 2.”
In the trial, subjects received a single intravenous injection of ABO-102 to facilitate systemic delivery of a corrective copy of the gene associated with onset and progression of MPS IIIA. Subjects were evaluated at multiple time points post-injection for safety assessments and signals of biopotency and clinical activity.
“Results thus far from the ongoing ABO-102 clinical trial support the tolerability of a systemically delivered AAV approach for the treatment of lysosomal storage diseases,” stated Timothy J. Miller, Ph.D., president and CEO of Abeona Therapeutics. “The large reductions in heparan sulfate in both CSF and urine, significant organ changes and demonstration of neurocognitive benefits represent important findings that support our path to regulatory guidance later this year. Importantly, the FDA recently allowed the lowering of the enrollment age to include subjects as young as 6 months, supporting the clinical paradigm of treating subjects earlier in their disease manifestation.”
Select updated data from the presentation are highlighted below, and the full presentation is available here: https://abeonatherapeutics.com/investors/
Biopotency Assessments: ABO-102 continues to demonstrate significant dose-dependent and time-dependent responses in measured biomarkers, including rapid and sustained reductions of heparan sulfate (HS), the sugar molecule that is the hallmark of MPS IIIA, in the cerebral spinal fluid (CSF) and urine.
CSF heparan sulfate:
• Day 180 assessment
o Cohort 2 (n=3) demonstrated a reduction of 57.1% +/- 7.14%
• Day 30 assessment
o Cohort 3 (n=3) demonstrated a reduction of 64.4% +/- 2.2%
Urine heparan sulfate (HS):
• Day 30 assessment
o Cohort 3 (n=3) demonstrated a reduction of 91.9% +/-1.5%
o Cohort 2 (n=3) demonstrated a reduction of 54.0% +/- 18.0%
• Day 60 assessment
o Cohort 3 (n=4) demonstrated a reduction of 73.4% +/- 13.5%
o Cohort 2 (n=3) demonstrated a reduction of 65.2% +/- 5.5%
• Day 90 assessment
o Cohort 3 (n=1) demonstrated a reduction of 94.9 %
o Cohort 2 (n=3) demonstrated a reduction of 63.1%+/- 8.2%
• Day 180 assessment
o Cohort 2 (n=2) demonstrated a reduction of 55.0% +/- 5.0%
o Cohort 1 (n=2) demonstrated a reduction of 29.2% +/- 35.6%
Biophysical Assessments: A separate natural history study (Truxal et. al., 2016, Mol. Genet. Metab.) of MPS IIIA demonstrated that subjects showed an average 220% increase in liver volume at baseline. Result from the Phase 1/2 clinical trial for ABO-102 demonstrate durable biophysical reductions of disease burden including reductions in liver volume.
Cognitive Assessments: ABO-102 continues to show evidence of stabilization or improvement in cognitive function at six months in Cohort 2 and one year in Cohort 1.
• Two of the three treated patients from Cohort 2 showed evidence of improvement in the Leiter-R non-verbal IQ and stabilized Vineland (adaptive behavior) scales.
Safety Assessments: ABO-102 is well-tolerated in all subjects to date, with no drug-related serious adverse events (SAE) reported through over 3,100 cumulative days post-injection.
ABO-102 has been granted Rare Pediatric Disease Designation and Fast Track Designation in the U.S. and Orphan Product Designation in both the USA and the European Union.
Investor Conference Call Details: The Company will provide investors with an update on the reported data tomorrow, Friday, February 9th, at 09:00 am (Eastern). Interested parties are invited to participate in the call by dialing 877-407-9210 (toll free domestic) or 201-689-8049 (international). The webcast, as well as presentation materials, will be accessible by visiting: https://www.webcaster4.com/Webcast/Page/1818/24557.
About Abeona: Abeona Therapeutics Inc. is a clinical-stage biopharmaceutical company developing cell and gene therapies for life-threatening rare genetic diseases. Abeona’s lead programs include EB-101 (gene-corrected skin grafts) for recessive dystrophic epidermolysis bullosa (RDEB), ABO-102 (AAV-SGSH), an adeno-associated virus (AAV) based gene therapy for Sanfilippo syndrome type A (MPS IIIA) and ABO-101 (AAV-NAGLU), an adeno-associated virus (AAV) based gene therapy for Sanfilippo syndrome type B (MPS IIIB). Abeona is also developing ABO-201 (AAV-CLN3) gene therapy for juvenile Batten disease (JNCL), ABO-202 (AAV-CLN1) for treatment of infantile Batten disease (INCL), EB-201 for epidermolysis bullosa (EB), ABO-301 (AAV-FANCC) for Fanconi anemia (FA) disorder and ABO-302 using a novel CRISPR/Cas9-based gene editing approach to gene therapy for rare blood diseases. In addition, Abeona is developing a proprietary vector platform, AIM™, for next generation product candidates. For more information, visit www.abeonatherapeutics.com.
Abeona Therapeutics Reports Top-Line Data from Phase 1/2 Gene Therapy Trial in MPS IIIA
Abeona Therapeutics Reports Top-Line Data from Phase 1/2 Gene Therapy Trial in MPS IIIA
• ABO-102 results presented at WORLDSymposium for Lysosomal Diseases show significant time- and dose-dependent reduction of underlying disease pathology, including decreased CSF and urine GAGs (HS fragments) and diminished liver volumes
• Evidence of cognitive benefit at six months post treatment in Cohort 2 and at one year in Cohort 1
• Company receives FDA allowance to lower enrollment age to six months
• Company to host investor conference call Friday, February 9th at 9:00 am ET; dial in 877-407-9210 (toll free domestic) or 201-689-8049 (international)
NEW YORK and CLEVELAND, Feb. 08, 2018 (GLOBE NEWSWIRE) — Abeona Therapeutics Inc. (NASDAQ:ABEO), a leading clinical-stage biopharmaceutical company focused on developing novel cell and gene therapies for life-threatening rare genetic diseases, today announced updated clinical data from the ongoing Phase 1/2 trial for ABO-102 (AAV-SGSH), the company’s investigational gene therapy for the treatment of Sanfilippo syndrome Type A (MPS IIIA), a rare autosomal-recessive lysosomal storage disease. The results demonstrate robust and durable clinical effects achieved throughout various timepoints post-administration. To date, 10 patients have been dosed with a single intravenous injection of ABO-102. Results were reported today during the WORLDSymposium for Lysosomal Diseases being held this week in San Diego, CA.
“MPS IIIA is a profound and deadly lysosomal storage disease with no approved treatments available. The encouraging clinical data reported today provide strong additional support for a whole-body treatment approach involving intravenous delivery of an AAV to drive expression of the SGSH enzyme in all organs of the body, with an emphasis on expression in the central nervous system,” stated Kevin M. Flanigan, M.D., principal investigator of the trial, director of the Center for Gene Therapy at Nationwide Children’s Hospital and professor of pediatrics and neurology at The Ohio State University College of Medicine. “We are especially pleased to see sustained decreases in CSF heparan sulfate in all subjects post-injection, along with positive signals of neurocognitive activity in Cohorts 1 and 2.”
In the trial, subjects received a single intravenous injection of ABO-102 to facilitate systemic delivery of a corrective copy of the gene associated with onset and progression of MPS IIIA. Subjects were evaluated at multiple time points post-injection for safety assessments and signals of biopotency and clinical activity.
“Results thus far from the ongoing ABO-102 clinical trial support the tolerability of a systemically delivered AAV approach for the treatment of lysosomal storage diseases,” stated Timothy J. Miller, Ph.D., president and CEO of Abeona Therapeutics. “The large reductions in heparan sulfate in both CSF and urine, significant organ changes and demonstration of neurocognitive benefits represent important findings that support our path to regulatory guidance later this year. Importantly, the FDA recently allowed the lowering of the enrollment age to include subjects as young as 6 months, supporting the clinical paradigm of treating subjects earlier in their disease manifestation.”
Select updated data from the presentation are highlighted below, and the full presentation is available here: https://abeonatherapeutics.com/investors/
Biopotency Assessments: ABO-102 continues to demonstrate significant dose-dependent and time-dependent responses in measured biomarkers, including rapid and sustained reductions of heparan sulfate (HS), the sugar molecule that is the hallmark of MPS IIIA, in the cerebral spinal fluid (CSF) and urine.
CSF heparan sulfate:
• Day 180 assessment
o Cohort 2 (n=3) demonstrated a reduction of 57.1% +/- 7.14%
• Day 30 assessment
o Cohort 3 (n=3) demonstrated a reduction of 64.4% +/- 2.2%
Urine heparan sulfate (HS):
• Day 30 assessment
o Cohort 3 (n=3) demonstrated a reduction of 91.9% +/-1.5%
o Cohort 2 (n=3) demonstrated a reduction of 54.0% +/- 18.0%
• Day 60 assessment
o Cohort 3 (n=4) demonstrated a reduction of 73.4% +/- 13.5%
o Cohort 2 (n=3) demonstrated a reduction of 65.2% +/- 5.5%
• Day 90 assessment
o Cohort 3 (n=1) demonstrated a reduction of 94.9 %
o Cohort 2 (n=3) demonstrated a reduction of 63.1%+/- 8.2%
• Day 180 assessment
o Cohort 2 (n=2) demonstrated a reduction of 55.0% +/- 5.0%
o Cohort 1 (n=2) demonstrated a reduction of 29.2% +/- 35.6%
Biophysical Assessments: A separate natural history study (Truxal et. al., 2016, Mol. Genet. Metab.) of MPS IIIA demonstrated that subjects showed an average 220% increase in liver volume at baseline. Result from the Phase 1/2 clinical trial for ABO-102 demonstrate durable biophysical reductions of disease burden including reductions in liver volume.
Cognitive Assessments: ABO-102 continues to show evidence of stabilization or improvement in cognitive function at six months in Cohort 2 and one year in Cohort 1.
• Two of the three treated patients from Cohort 2 showed evidence of improvement in the Leiter-R non-verbal IQ and stabilized Vineland (adaptive behavior) scales.
Safety Assessments: ABO-102 is well-tolerated in all subjects to date, with no drug-related serious adverse events (SAE) reported through over 3,100 cumulative days post-injection.
ABO-102 has been granted Rare Pediatric Disease Designation and Fast Track Designation in the U.S. and Orphan Product Designation in both the USA and the European Union.
Investor Conference Call Details: The Company will provide investors with an update on the reported data tomorrow, Friday, February 9th, at 09:00 am (Eastern). Interested parties are invited to participate in the call by dialing 877-407-9210 (toll free domestic) or 201-689-8049 (international). The webcast, as well as presentation materials, will be accessible by visiting: https://www.webcaster4.com/Webcast/Page/1818/24557.
About Abeona: Abeona Therapeutics Inc. is a clinical-stage biopharmaceutical company developing cell and gene therapies for life-threatening rare genetic diseases. Abeona’s lead programs include EB-101 (gene-corrected skin grafts) for recessive dystrophic epidermolysis bullosa (RDEB), ABO-102 (AAV-SGSH), an adeno-associated virus (AAV) based gene therapy for Sanfilippo syndrome type A (MPS IIIA) and ABO-101 (AAV-NAGLU), an adeno-associated virus (AAV) based gene therapy for Sanfilippo syndrome type B (MPS IIIB). Abeona is also developing ABO-201 (AAV-CLN3) gene therapy for juvenile Batten disease (JNCL), ABO-202 (AAV-CLN1) for treatment of infantile Batten disease (INCL), EB-201 for epidermolysis bullosa (EB), ABO-301 (AAV-FANCC) for Fanconi anemia (FA) disorder and ABO-302 using a novel CRISPR/Cas9-based gene editing approach to gene therapy for rare blood diseases. In addition, Abeona is developing a proprietary vector platform, AIM™, for next generation product candidates. For more information, visit www.abeonatherapeutics.com.
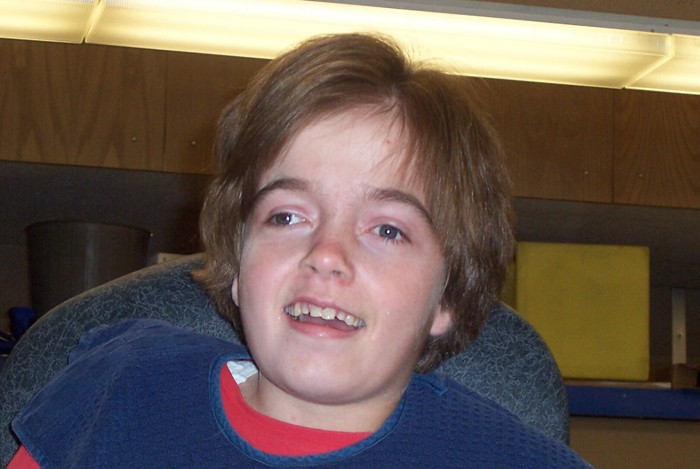
Kirby Wilson
Brad and I formed this Foundation because of our four-year-old daughter, Kirby, the disease she was afflicted with, and our hope that Sanfilippo and its devastating progression would not manifest itself within her — our beautiful bundle of joy. The solution seemed simple. Raise funds to enable researchers to advance and expand their work to find a cure. We chose to fight this disease.
Now 24 years later, although Sanfilippo has taken its toll on Kirby, she continues to defy her affliction with seemingly endless resolve and still an occasional smile for us to cherish. A smile that brings us reassurance of her comfort and delight in her moments of happiness — a reminder of her amazing grace. She is our hero.
As her battle continues, our dream of a cure could come true at Nationwide Children’s Hospital in Columbus, OH. Drs. Haiyan Fu and Douglas McCarty from Nationwide’s Center for Gene Therapy, along with physicians from the Children’s Hospital, are ready to bring Drs. Fu and McCarty’s 19-year-old gene therapy program to human clinical trial. Joining forces with other families and foundations, we hope to raise the necessary funds to bring their work to fruition.
The price to fund these studies and continue to support other valuable research might seem insurmountable. But all Brad and I have to do is remember what doctors first told us 24 years ago: Nothing can be done. Enjoy her while you have her.
Then our thoughts turn to Kirby. Her fortitude must not be in vain. No matter what the future holds for her, our mission is still for her. Because it is her story that is repeated in hundreds of families around the world, hundreds of stories shaped by one cure. And it has been your faithful belief in our mission that has allowed Kirby’s story to be written and your unwavering support of the Foundation that has helped research to reach this level. It can be done.
We ask that you think of Kirby, and in her honor, continue to support The Children’s Medical Research Foundation and its mission of a cure.
With gratitude,
Sue and Brad Wilson


