December, 2022. Below is an update from Dr. Mitchell Drumm, who is leading the team working with Sanfilippo at Case Western’s Research Institute for Children’s Health. As Dr. Drumm previously stated when talking about his enthusiasm to add Sanfilippo, “Quite simply, this is why the Research Institute for Children’s Health exists. Our researchers may be scientists, but they are also parents.” We are excited by the potential the Research Institute’s work holds for children afflicted today. As Sue states, “Being a small part of Dr. Drumm and his team’s efforts to help children afflicted with Sanfilippo is an honor and privilege. Dr. Drumm’s compassion for children is only matched by the integrity of his work.”
Our wish is that his work encourages others within the Sanfilippo community to speak with him, learn more about his methods and goals, and join in our efforts. We will be posting a video here within the next month by Dr. Drumm showing his team at work in the laboratory, so you can see, firsthand, the inspiring work that is being done.
Dr. Mitch Drumm
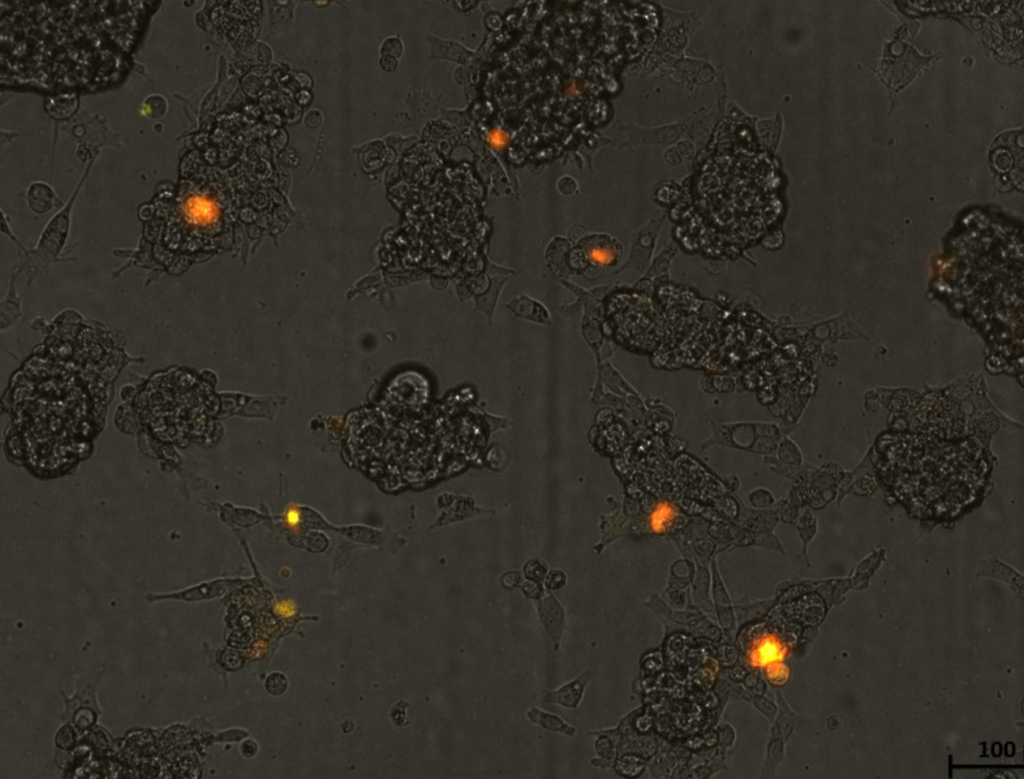
My lab is working on two strategies to deal with mutations in NAGLU, the gene that causes Sanfilippo. One is to actually repair the mutation in a patient’s cells and the other is to add a functional copy of the gene to the cells. The NAGLU enzyme, once made by the cell, must reach a part of the cell called the lysosome where it does its part to break down heparan sulfate. Mitch has used the CRISPR/Cas9 system to engineer cells lacking the NAGLU enzyme to model Sanfilppo and is now testing the gene repair and gene supplementation strategies. So far, the results are promising: Adding back a normal copy of the gene provides enzyme activity to the cells, opening the door to testing the strategy in mice, looking not only for enzyme activity, but also positive effects on the disease.” Mitch explains that his lab has added to the NAGLU enzyme part of a gene from jellyfish that makes the NAGLU enzyme glow, or fluoresce, so they can actually see it when using a special microscope. His lab introduces the fluorescent version of NAGLU into Sanfilippo cells, just as they would in a gene therapy protocol, so they can not only see if the gene provides the needed NAGLU enzyme, but also visualize where it goes and how long it lasts. In the picture, you can see cells grown in a dish (the grey globs that look like a bunch of frog eggs) in which the NAGLU-jellyfish protein is being made, as the ones that successfully took up the NAGLU gene glow orange.
December, 2021. We continue to look for ways to accelerate research to ensure that hope shines bright for children afflicted worldwide. With our commitment to Dr. Mitch Drumm and Case Western Reserve’s Research Institute for Children’s Health, we feel we are doing just that.
The Institute’s focus is on Sanfilippo and other rare diseases, and it uses proven, successful strategies while cultivating research expertise by championing young scientists – challenging them to be bold in the lab with their new and exciting ideas. We can think of no better way of honoring our dear Kirby’s legacy of a cure and impacting the time so precious to children living with rare diseases today.
Dr. Mitchell Drumm
I have been working on genetic diseases for nearly 40 years and over that time have seen advancements in science that change our understanding, provide new tools for us to use and create breakthroughs in medical treatments. These advances are almost always unpredictable. As Sue alluded, time is of the essence, but it is also the one variable we can’t control. My approach is to make better use of the time we have by increasing the number of people trying to tackle problems. The Foundation is helping me to engage young, energized scientists to work on Sanfilippo, not only accelerating the research toward treatments, but also ensuring that it is sustainable. We are enlisting the next generation of scientific minds so that the process will continue until we have reached our goals.
Our work in rare, genetic diseases focuses on fundamental problems, from identifying the gene involved, figuring out which cells in the body are contributing to the disease and then devising strategies to repair or replace the gene in the appropriate places. For Sanfilippo, we know the gene and think we know the cells, thanks to work supported by the Foundation, but the latter steps need to be worked out. To do so, we rely on cell culture systems, where we grow human cells in a petri dish to see if we can restore a gene or enzyme activity and then apply what we’ve learned to mouse models to determine if we can do the gene repair or enzyme restoration in the appropriate cells to an extent that will have clinical effect.
We have generated versions of the NAGLU enzyme to which we joined a fluorescent protein so that we could track the protein in cells we wished to repair or otherwise provide NAGLU enzyme activity. These “fusion proteins” have worked out well and seem to end up in the same location in cells as the native enzyme. They are quite readily detected using our high-powered microscope that detects fluorescence.
Our most current challenge is that we could not find in our repertoire of laboratory cells any that don’t already make large amounts of the NAGLU enzyme, preventing us from seeing if our fusion protein maintains enzymatic activity. To solve this, we are using a gene editing technique called CRISPR/Cas9 to create a model of Sanfilippo by disrupting the NAGLU gene. This is also one of the technologies we will eventually test for its ability to repair mutations causing Sanfilippo. We are currently sequencing the DNA of the cells and have several promising candidates to use for our studies.
Once these laboratory cell systems are vetted, our next steps are to see if we can restore NAGLU activity to them through gene replacement (putting in a normal copy of the gene) or gene repair by editing. From there, we will begin our work on delivering these therapeutic systems into various cells in mice. While we never feel we are moving fast enough, we do feel we’re moving in the right direction and are extremely thankful to the Foundation for including us in their mission.
December, 2020. The Foundation is thrilled to announce its two-year award to fund a postdoctoral fellowship: Gene Therapy for Sanfilippo Syndrome at the Research Institute for Children’s Health at Case Western Reserve University in Cleveland, Ohio, under the leadership of Dr. Mitchell Drumm.
Dr. Drumm earned his doctoral degree in the laboratory of Francis Collins, M.D., PhD, with whom he co-discovered the gene that causes cystic fibrosis (CF). Dr. Drumm has been involved in the therapeutic development processes for cystic fibrosis ever since and in 2015 launched the Research Institute for Children’s Health with the philosophy that one take the successful strategies learned in CF and apply them to other genetic disorders, moving more quickly and efficiently by not reinventing the wheel. Dr. Drumm is excited to engage in gene therapy for Sanfilippo because of the transformative changes in genetic therapy technology now available. His lab and colleagues have developed a pipeline approach to move therapeutics to the clinic in other rare, genetic disorders using gene-based therapies.
Dr. Drumm’s implementation of laboratory-to-clinic research programs for rare, genetic disorders patterned after the CF approach is exciting and now provides a fresh look at gene therapy for Sanfilippo, revitalizing hope for families and children afflicted.
Dr. Drumm has described for us that his laboratory has created mouse models that allow easier and stronger detection of successful steps in the gene therapy process. He says that current technologies don’t allow us to look for the enzyme in individual cells of the body, or how long it lasts. The cells of the mice they’ve developed actually light up if the gene therapy approach reaches them, making them straightforward to find. Dr. Drumm explains “Our mice, that let us see where the gene therapy particles go, how many cells they enter, and how long they last, will provide a guide when we compare different gene therapy approaches in the Sanfilippo mouse that the Foundation had the foresight to fund. We have teamed up with AAV gene therapy experts as the first approach we will try, but are set up to evaluate any system one wishes to try, or even combinations of systems. The support from the Foundation will allow a talented postdoctoral fellow in my lab, Karen Schelde, PhD, who has extensive experience with mice in this regard, to test the gene therapy strategies.” Dr. Drumm is very excited for his lab to work on Sanfilippo. “Quite simply, this is why the Research Institute for Children’s Health exists,” he says. “Our researchers may be scientists, but they are also parents.”


